FermCalc - Alcohol & Solids Content Calculators
Back to topHydrometer Temperature Corrections
FermCalc allows hydrometer readings to be corrected for temperature in all three of the alcohol content calculation methods. The corrections are functions of both the alcohol and solids content of the liquids. The alcohol correction is determined from the OIML formula (OIML, 1973). The solids correction is determined from a fit of tables published in the AOAC Official Methods of Analysis (Williams, 1984) for correcting hydrometer btix readings. These tables have a reference temperature of 20°C, so first the readings need to be corrected for the hydrometer calibration temperature if it's different from 20°C.
The SG measured with a hydrometer is the ratio of the density of the liquid divided by the density of water at the hydrometer calibration temperature, or:
sgaTc = ρT/ρwTc (1)
where
sgaTc = apparent SG measured with a hydrometer with calibration
temperature Tc
ρT = liquid density at temperature T, kg/L
ρwTc = water density at temperature Tc, kg/L
To correct the reading to a reference temperature of 20°C, we simply need to multiply the hydrometer reading by the ratio of the water density at the old calibration temperature to the water density at 20°C, or:
sga20 = sgTc (ρwTc/ρw20) (2)
where
sga20 = apparent SG corrected to a reference temperature of 20°C
ρw20 = water density at 20°C, kg/L
Water densities are calculated using the OIML formula assuming 0% alcohol.
After correcting for the hydrometer calibration temperature, we can correct for the sample temperature. The coefficient of thermal expansion for aqueous ethanol-sucrose solutions, which determines the change in density for a given change in temperature, depends on both the ethanol and sucrose levels, but mainly on the ethanol level (Espejo and Armada, 2011). To correct the measured SG, FermCalc determines a composite correction factor by first calculating three density correction factors as follows:
dw = ρw20 / ρwT (3) da = ρa20 / ρaT (4) ds = ρs20 / ρsT (5)
where
dw = density correction factor for pure water
da = density correction factor an ethanol-water solution with the same alcohol
content as the liquid
ds = density correction factor a sucrose-water solution with the same sucrose
content as the liquid
ρw20 = water density at 20°C, kg/L
ρa20 = ethanol-water solution density at 20°C, kg/L
ρs20 = sucrose-water solution density at 20°C, kg/L
ρwT = water density at the measurement temperature, kg/L
ρaT = ethanol-water solution density at the measurement temperature, kg/L
ρsT = sucrose-water solution density at the measurement temperature, kg/L
The corrected SG sgc20 is then calculated as:
sgc20 = sga20dads/dw (6)
The densites for water and ethanol-water solutions are based on the OIML formula. The change in density of sucrose-water solutions with temperature is based on the following curve fit of the AOAC brix correction tables:
B = Ba + [a(T - 20)2 + b(T - 20)] (7)
where:
B = corrected Brix
Ba = apparent Brix at temperature T
T = measurement temperature, °C
a = 1.4525·10-7Ba2 - 2.5256·10-5Ba + 1.2495·10-3
b = -6.6927·10-6Ba2 + 9.6012·10-4Ba + 4.4174·10-2
The plot below compares the AOAC data with the calculated Brix corrections from equation (7) above.
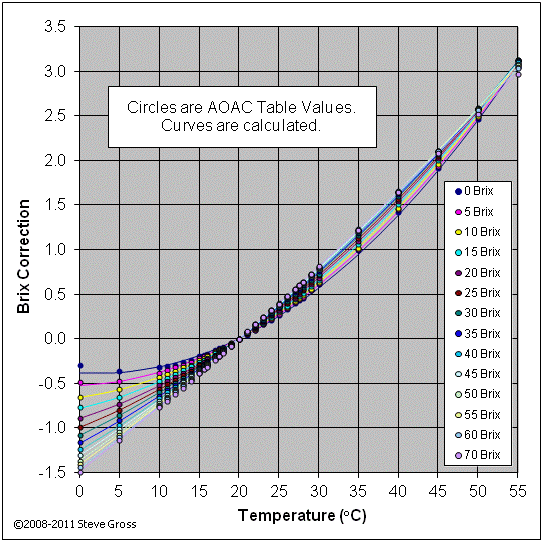
The temperature corrections are valid in the temperature range from 0°C to 40°C, which is the overlapping range of validity between the OIML formula and the AOAC tables. Some of the alcohol content calculation methods below are based on SG values at 60°F instead of 20°C, in which cases the appropriate densities are substituted into the above equations. FermCalc only reports the SG values corrected to 20°C, so the actual SG values being used in the equations will be slightly different than the reported SG values in these cases.
Back to topHydrometer SG Drop Calculator
Introduction
The Hydrometer SG Drop Calculator estimates the alcohol content of a wine from two specific gravity (SG) measurements: one obtained prior to fermentation, and one obtained after fermentation is complete. Four different calculation methods are used to estimate alcohol content:
The calculator will adjust the hydrometer readings for temperature if the measurement temperatures and the hydrometer calibration temperatures are provided. If the entered SG values are already corrected for temperature, or if temperature corrections are not desired, simply enter 20°C (68°F) in all of the temperature fields and no corrections will be made.
This calculation is only valid for SG or density measurements obtained with a hydrometer or some other device for directly measuring SG or density. It does not work with refractometer readings because of the obscuration effect of alcohol on refractive index. The final SG measurement must be taken prior to any post-fermentation sweetening. If any sweetener is added during fermentation after the initial SG measurement is taken, the initial SG measurement must be adjusted accordingly.
Dr. William Honneyman (1966) compared alcohol levels calculated using the SG drop method to the distillation data of Thorpe & Brown (1914) and found that it gives results "reasonably comparable with tests by distillation, provided graded factors suited to each drop in gravity are used". While he did not recommend a specific way to calculate the graded factors, he provided an important validation of the approach in general.
Back to topInput Field Definitions
Hydrometer SG Reading – The initial and final hydrometer SG readings. The initial reading should be taken prior to fermentation, and the final SG reading should be taken after fermentation is complete. Range: 0.77193 to 1.55454
SG Reading Temperature – The temperatures of the must and wine samples at the time of the initial and final SG readings. Range: 0°C (32°F) to 40°C (104°F)
Calibration Temperature – The hydrometer calibration temperature(s) of the hydrometer(s) used to take the initial and final SG readings. Range: 0°C (32°F) to 40°C (104°F)
Output Field Definitions
Corrected SG (20°C/20°C) – The temperature-corrected initial and final SG values.
Alcohol Content – The alcohol content of the wine calculated from the difference between the initial and final corrected SG values using the four calculation methods listed above.
True Brix (Solids Content) – The total solids content of the wine calculated from the final corrected SG value and the average alcohol content of the four calculation methods.
Calculation Details
Berry Method
This is the most commonly used SG drop method, and is described on pages 79-80 of First Steps in Winemaking by C. J. J. Berry (1987). It estimates the alcohol content by dividing the drop in SG by the constant 0.00736, or:
av = (sgi - sgf) / 0.00736 (8)
where
av = alcohol content, % by volume
sgi = initial SG
sgf = final SG
Ritchie Products Co. (2004) claims to have compared the results of equation (8) to the results of gas chromatography for a wide range of wines, and found that the results were within 0.3% vol/vol.
This method has a temperature basis of 15.56°C (60°F).
Back to topDuncan & Acton Method
This method is described on pages 64-66 of Progressive Winemaking by Peter Duncan and Bryan Acton (1967). The Duncan & Acton method calculates the alcohol content from the initial and final specific gravities divided by a factor F that is a function of the corrected initial SG. The equations are as follows.
av = 1000(sgi - sgf) / F (9) F = 7.75 - 3000(sgc - 1.0) / 800 (10) sgc = sgi - 0.007 (11)
where
av = alcohol content, % by volume
sgi = initial SG
sgf = final SG
F = conversion factor
sgc = initial SG corrected for non-sugar solutes
Combining equations (9) through (11) above yields the following equation:
av = 1000(sgi - sgf) / [7.75 - 3.75(sgi - 1.007)] (12)
This method has a temperature basis of 15.56°C (60°F).
Back to topBalling Method
The Balling method is normally used for beer but gives results that agree very well with the other methods. The equations used in FermCalc were taken from Michael Hall's article "Brew by the Numbers" in the Summer 1995 issue of Zymurgy magazine. In the original equations, specific gravities are expressed as degrees Plato, which FermCalc treats as being the equivalent as degrees Brix. The method requires the calculation of a parameter called "Real Extract", which is an estimate of the residual solids content after fermentation has finished, as follows:
q = 0.22 + 0.001Bi (13)
RE = (q·Bi + Bf) / (1 + q) (14)
where
q = attenuation coefficient
RE = real extract
Bi = initial Brix
Bf = final Brix
The alcohol content (% by weight) is then calculated as:
aw = (Bi - RE) / (2.0665 - 0.010665Bi) (15)
where aw is the alcohol content in % by weight.
The result of equation (15) is then converted to % alcohol by volume as described here.
This method has a temperature basis of 17.5°C (63.5°F).
Back to topCutaia, Reid, & Speers Method
Cutaia, Reid & Spears (2009) analyzed data from 532 beers to develop equation (12) below relating alcohol content to the initial and final specific gravities.
aw = (Bi - Bf)(0.372 + 0.00357Bi) (16)
The alcohol content of the beers ranged from 3% to 7% by weight (approx. 3.8% to 8.7% by vol.). As with the Balling equation above, the specific gravities for the analyzed beers were expressed in degrees Plato, which is assumed by FermCalc to be the same as degrees Brix.
The result of equation (16) is then converted to % alcohol by volume as described here.
This method has a temperature basis of 20°C (68°F).
Back to topEstimation of Solids Content (True Brix)
After we know the alcohol content, we can estimate true Brix, which represents solids content in % by weight, by using the model developed by James Hackbarth (2011), which is described below. This is done by treating the specific gravity SG and alcohol content A as known values and iteratively solving equations (38) through (44) below for the true Brix E. FermCalc uses the alcohol content calculated by the Duncan & Acton method for this calculation.
Back to topHydrometer & Refractometer Calculator
Introduction
The Hydrometer and Refractometer Calculator estimates the alcohol content of a finished wine from a refractometer reading and a hydrometer specific gravity (SG) reading. Four different calculation methods are used to estimate alcohol content:
All of the methods are designed to be used after fermentation, but they should be able to yield reasonable estimates of alcohol content during fermentation as long as there is enough alcohol to affect the measurements and the sample is degassed enough that the measurements are not affected by dissolved CO2.
Input Field Definitions
Refractometer Reading – The refractometer reading for the wine after fermentation. Range: 0° to 100°Brix
Hydrometer SG Reading – The hydrometer SG reading for the wine sample. Range: 0.77193 to 1.55454
SG Reading Temperature – The temperature of the wine sample at the time of the SG reading. Range: 0°C (32°F) to 40°C (104°F)
Calibration Temperature – The hydrometer calibration temperature of the hydrometer used to take SG reading. Range: 0°C (32°F) to 40°C (104°F)
Output Field Definitions
Corrected SG (20°C/20°C) – The temperature-corrected SG value.
Alcohol Content – The alcohol content of the wine calculated using the four calculation methods listed above.
True Brix – The total solids content of the wine calculated using the four calculation methods listed above.
Back to topCalculation Details
Rogerson & Symington Method
Rogerson & Symington (2006) developed a method to estimate alcohol content and residual solids (true Brix) based on refractometer and hydrometer readings on 35 port wines. In the words of the authors, "It is not applicable for the analysis of dry wines, whether fortified or not, which contain insufficient soluble solids for Baumé determination by hydrometer, and is yet to be evaluated for sweet table wines, such as sauternes." However it is included in FermCalc because many home winemakers seem to find it useful for monitoring fermentation progress and calculating alcohol content.
FermCalc first converts the hydrometer reading sg to degrees Baumé using the following equation.
Bé = 145 - 145/sg (17)
where Bé is degrees Baumé.
Alcohol content is then calculated as:
av = 1.646Ba - 2.703Bé - 1.794 (18)
where
av = alcohol content, % by volume
Ba = refractometer Brix reading (apparent Brix)
True Brix, Bt, which represents the estimated residual solids content in % by weight, is then calculated as:
Bt = Ba - 0.358av (19)
This method has a temperature basis of 20°C (68°F).
Back to topSon et al. Method
H. S. Son et al. (2009) developed the following six empirical equations based on refractometer, hydrometer, and alcohol content measurements on 30 wines before and during fermentation.
Bt = -0.352Bi + 1.264Ba + 2.006 (20) Bt = 0.201Bi + 0.782Bh - 0.921 (21) av = 0.967Bi - 0.766Ba - 5.793 (22) av = 0.625Bi - 0.457Bh - 3.814 (23) Bt = 0.529Ba + 0.457Bh - 0.344 (24) av = 0.833Ba - 0.996Bh + 3.927 (25)
where
av = alcohol content, % by volume
Bi = initial Brix reading
Ba = refractometer Brix reading (apparent Brix)
Bh = hydrometer Brix reading
Bt = true Brix (% solids by weight)
Equations (24) and (25) allow calculation of alcohol content and true Brix directly from hydrometer and refractometer readings. However, I found these equations to be inaccurate, yielding estimates of alcohol content that appear too high in the lower-alcohol range and too low in the upper range. Instead of using equations (24) and (25), I developed alternative equations from equations (20) through (23) that appear much more accurate. Combining equations (22) and (23) to eliminate Bi gives:
av = 1.400Ba - 1.292Bh + 0.197 (26)
Combining equations (20) and (21) to eliminate Bi gives:
Bt = 0.459Ba + 0.498Bh + 0.143 (27)
Equations (26) and (27) are used by FermCalc to calculate alcohol content and true Brix.
This method has a temperature basis of 20°C (68°F).
Back to topRoesener Method
This method was published online by Werner Roesener (2001) and is very popular among home winemakers, but there is no documentation regarding the derivation of the equations. My testing indicates that it yields results that are very similar to the other methods.
Simplifying the original equations we get:
av = 1.5184Ba + 365(1.0 - sg) (28) s = 2520(sg - 1.0) + 3.1853av (29)
where s is the dissolved solids content in g/L. FermCalc converts the solids content in g/L to true Brix in percent by weight using equation (30) below.
Bt = s/sg/10 (30)
This method has a temperature basis of 15.56°C (60°F).
Back to topBarth & Race Method
This method was originally developed by Georg Barth (1905) in Germany for analyzing beers. The original equations are:
aw = 759.8(ri - 1.3330) - 292.3(sg - 1.0) (31) Bt = 336.6(ri - 1.3330) + 130.3(sg - 1.0) (32)
Where ri is the measured refractive index. The equations were later modified by J. Race (1908) to yield more accurate results for beers with alcohol contents greater than 4.5% by weight.
aw = 778(ri - 1.3330) - 290(sg - 1.0) (33) Bt = 350(ri - 1.3330) + 130(sg - 1.0) (34)
FermCalc uses equations (33) and (34) to calculate alcohol content and true Brix because they were intended for higher alcohol concentrations and might be more applicable for winemaking calculations. The result of equation (32) is converted to % alcohol by volume as described here.
This method has a temperature basis of 15.56°C (60°F).
Back to topBoiling (Spirit-Indication) Calculator
Introduction
The Boiling (Spirit Indication) Calculator estimates the alcohol content of a wine from two specific gravity (SG) measurements: one obtained on a sample of the finished wine, and one obtained on a sample of the wine in which the alcohol has been boiled off and distilled water has been added to restore it to its pre-boiled volume. Four different calculation methods are used to estimate alcohol content:
The calculator will adjust the hydrometer readings for temperature if the measurement temperatures and the hydrometer calibration temperatures are provided. If the entered SG values are already corrected for temperature, or if temperature corrections are not desired, simply enter 20°C (68°F) in all of the temperature fields and no corrections will be made.
Input Field Definitions
Hydrometer SG Reading – The initial and final hydrometer SG readings. The initial reading should be taken prior to boiling, and the final SG reading should be taken after boiling off the alcohol and restoring the sample to its original volume with distilled water. Range: 0.77193 to 1.55454
SG Reading Temperature – The temperatures of the samples at the time of the initial and final SG readings. Range: 0°C (32°F) to 40°C (104°F)
Calibration Temperature – The hydrometer calibration temperature(s) of the hydrometer(s) used to take the initial and final SG readings. Range: 0°C (32°F) to 40°C (104°F)
Output Field Definitions
Corrected SG (20°C/20°C) – The temperature-corrected initial and final SG values.
Alcohol Content – The alcohol content of the wine calculated from the difference between the initial and final corrected SG values using the four calculation methods listed above.
True Brix (Solids Content) – The total solids content of the wine calculated from the initial and final corrected SG values.
Calculation Details
This method was first proposed by M. E. Tabarié in 1830 as a simplified alternative to the distillation procedure. It is based on the principle that alcohol causes the same depression in SG in wine as it does in pure water.
The method involves evaporating (boiling off) a portion of the wine sample until all of the alcohol is evaporated, and then replacing the evaporated volume with distilled water. The difference between the specific gravities of the wine and the volume-corrected residue are then used to estimate the SG of the distillate, which represents the SG of a pure water/ethanol mixture, from which the alcohol content can be estimated. The experimental procedure is summarized below.
- Measure the SG (sgw) of the wine to be tested.
- Take a sample of about 250-500 mL (1-2 cups) of the wine and boil the sample down to approximately half of its original volume to drive off all of the alcohol.
- Allow the boiled residue to cool to room temperature.
- Add distilled water to the residue until the total volume is restored to the original sample volume.
- Measure the SG of this volume-corrected residue sgr, which will be greater than sgw because the alcohol has been replaced by water.
It is recommended that a narrow-range hydrometer be used for the SG measurements since small errors in these measurements can result in large errors in the results.
In addition to estimating the alcohol content, we can also calculate the solids content (true Brix) of the wine from the SG of the volume-corrected residue. First we just need to convert the residue SG measurement to a Brix value by using the Brix conversion equation. This conversion yields the solids content in % by weight of the residue. We then need to convert this value to the solids content in % by weight of the wine by multiplying by the ratio of specific gravities, or:
Bt = Btr·sgr/sgw (35)
where
Bt = true Brix (solids content) of wine, % by weight
Btr = true Brix (solids content) of the volume-corrected residue, % by weight
sgw = SG of wine
sgr = SG of the volume-corrected residue
The four methods that FermCalc uses to calculate the alcohol content from the SG measurements are described below.
Back to topTabarié (Division) Method
Tabarié originally proposed estimating the SG of the distillate from the ratio of the wine and residue specific gravities, or:
sgd = sgw/sgr (36)
where
sgd = SG of distillate
sgw = SG of wine
sgr = SG of the volume-corrected residue
FermCalc determines the alcohol content in % by volume from sgd by using the OIML formula (OIML, 1973).
This method has a temperature basis of 20°C (68°F).
Back to topBlunt (Subtraction) Method
T. P. Blunt (1891) suggested that the Tabarié division formula always underestimates alcohol content, and suggested calculating sgd from the difference between sgr and sgw instead of the ratio, or.
sgd = 1.0 - (sgr - sgw) (37)
FermCalc uses the OIML formula to estimate alcohol content in % by volume from the results of equation (37).
S. Harvey (1892) presented experimental results suggesting that Blunt's formula is more accurate than Tabarié's. However, a few pages later in the same issue of The Analyst, A. H. Allen presented data for sugar and alcohol solutions ranging from 24% to 53% alcohol by weight suggesting that the Tabarié formula was more accurate. While Blunt is generally credited with showing that the subtraction formula is more accurate than the division formula, the subtraction formula was clearly in use well before Blunt wrote his paper (Mulder, 1857).
This method has a temperature basis of 20°C (68°F).
Back to topHonneyman Method
This method is described on pages 124-126 of The Art of Making Wine (Anderson & Hull, 1970), and is attributed to the researches of Dr. William Honneyman (1966). This method is really identical to the Blunt subtraction method described above, so it might seem redundant to include it here. The main difference is that this method uses Dr. Honneyman's table below to estimate the alcohol content, and in the Blunt method we're using the OIML equation instead of a table.
| sgr - sgw | Alcohol Content (% vol/vol) |
sgr - sgw | Alcohol Content (% vol/vol) |
sgr - sgw | Alcohol Content (% vol/vol) |
|---|---|---|---|---|---|
| 0.0000 | 0.0 | 0.0090 | 6.4 | 0.0180 | 14.1 |
| 0.0015 | 1.0 | 0.0100 | 7.2 | 0.0190 | 15.1 |
| 0.0020 | 1.3 | 0.0110 | 8.0 | 0.0200 | 16.0 |
| 0.0030 | 2.0 | 0.0120 | 8.8 | 0.0210 | 17.0 |
| 0.0040 | 2.7 | 0.0130 | 9.7 | 0.0220 | 18.0 |
| 0.0050 | 3.4 | 0.0140 | 10.5 | 0.0230 | 19.0 |
| 0.0060 | 4.1 | 0.0150 | 11.4 | 0.0240 | 20.0 |
| 0.0070 | 4.9 | 0.0160 | 12.3 | 0.0250 | 21.0 |
| 0.0080 | 5.6 | 0.0170 | 13.2 | 0.0260 | 22.0 |
I obtained a copy of Dr. Honneyman's book and was able to determine that his table is based on the alcoholometric tables of Thorpe (1915), which have a temperature basis of 60°F. With this information I was able to extend his table to higher alcohol levels and increase the resolution. Now with the appropriate temperature correction the Honneyman method gives results that are nearly identical to the other boiling methods. There are very small differences because the temperature corrections are not exact and because the basis of the Thorpe tables is different from that of the OIML equation.
This method has a temperature basis of 15.56°C (60°F).
Back to topHackbarth Method
James Hackbarth (2009) showed that the Tabarié approach works reasonably well for dry wines and low-extract beers, but is inaccurate for beverages with higher alcohol and sugar concentrations due to solute-solute interactions that take place at the higher concentrations, an idea that was first proposed by Leonard (1897). Based on extensive laboratory experimentation and detailed analysis of the results, Hackbarth (2011) developed a new model for estimating the SG of a mixture from its sucrose (extract) and alcohol concentrations. Since the spirit-indication procedure gives us measurements of the wine SG and the extract concentration (true Brix), we can treat these as known quantities and use the Hackbarth model to solve for the alcohol content.
The Hackbarth model utilizes the following equations.
Z = -1.020733575·10-2E1A0.5 + 6.223951696·10-4E2A0.5
- 3.463023825·10-6E3A0.5 + 7.234029153·10-3E1A1
- 4.496851490·10-4E1A2 + 9.045618812·10-6E1A3
- 5.427265684·10-8E1A4 - 1.719663278·10-4E2A1
+ 2.302760700·10-9E3A3(38) Eb = E·100/(100 - A) + Z (39) Ab = A·100/(100 - E) + Z (40) SGWE = (100 - Eb)/[100/fe(Eb) - Eb/fe(100)] (41) SGWA = (100 - Ab)/[100/fa(Ab) - Ab/fa(100)] (42) SGW = SGWE · SGWA (43) SG = 100/[E/fe(100) + A/fa(100) + (100 - E - A)/SGW] (44)
where
Z = correction for solute interactions
A = alcohol concentration, % by weight
E = sucrose concentration (true Brix), % by weight
SG = SG of the ternary solution (wine)
Ab = alcohol concentration in the binary solution, % by weight
Eb = sucrose concentration (true Brix) in the binary solution, % by weight
fa(A) = 11th order polynomial for calculating SG from alcohol
concentration based on the OIML general formula (OIML, 1973)
fe(E) = 10th order polynomial for calculating SG from sucrose
concentration based on AOAC Plato tables
SGWA = SG of water in the binary solution of alcohol
SGWE = SG of water in the binary solution of sucrose
SGW = SG of water in the ternary solution (wine)
FermCalc solves equations (38) through (44) iteratively using the alcohol content calculated from the Blunt model as the initial estimate. The iteration loop is repeated until the difference between the value of SG calculated by equation (44) and the wine SG sgw is less than 10-8.
This method has a temperature basis of 20°C (68°F).
Back to topPotential Alcohol Calculator
Introduction
The Potential Alcohol Calculator estimates the alcohol content of a wine that will result from the complete fermentation of a must with a given initial specific gravity. Nine methods are used to estimate the potential alcohol content:
- Dubrunfaut Method
- Marsh Method
- Margalit Method
- Cooke & Lapsley Method
- CEC Method
- Pambianchi Method
- Duncan & Acton Method
- Honneyman Method
- User Defined Method
Most of these methods assume some sugar to alcohol conversion factor less than the theoretical maximum of 0.511 grams of alcohol per gram of sugar. Most of them also make an allowance for the presence of non-fermentable solids, most commonly assumed to be 30 g/L. The actual alcohol content will depend on the composition of the must and on the efficiency and completeness of the fermentation, so it is best measured after fermentation is finished.
Because of the approximate nature of these calculations, no temperature corrections are made to the SG or to the calculated alcohol contents. The SG measurement should be made near 20°C (68°F). For all of the methods below, conversions between SG and Brix are made using the SG to Brix conversion equation, which has a temperature basis of 20°C (68°F).
Input Field Definitions
Initial Must SG – The SG (20°C/20°C) of the must prior to the start of fermentation. Range: 1.0 to 1.5545 (0° to 100° Brix).
Fermentation Factor – This is the factor multiplied by Brix in the User Defined method. A range is not enforced, but this is usually in the range between 0.55 and 0.63.
Output Field Definitions
Potential Alcohol Content – The estimated alcohol content after complete fermentation.
Calculation Details
Dubrunfaut Method
This method is reported by Boulton et al (1999) and is attributed to Dubrunfaut.
ap = 0.059(2.66·Oe - 30) (45)
where
ap = potential alcohol content, % by weight
Oe = 1000(sgi - 1)
sgi = initial specific gravity of the must
Marsh Method
George Marsh (1958) proposed using a fermentation factor of 0.47 grams of alcohol per gram of sugar. The sugar content is calculated from the measured Brix minus a correction factor of 30 g/L (3 g/100 mL). The result is then divided by the density of alcohol to yield alcohol content in percent by volume. This yields the formula:
ap = 0.47(Bisgiρw - 3)/ρa (46)
where
Bi = Brix value corresponding to sgi
ρw = density of water = 0.9982 g/mL at 20°C
ρa = density of alcohol = 0.7892 g/mL at 20°C
Margalit Method
Margalit (2004) calculated that 1.75°Brix results in 1% alcohol by volume, resulting in the formula:
ap = 0.57Bi (47)
Cooke & Lapsley Method
Cooke & Lapsley (1988) proposed a formula very similar to that of Marsh (1958), the main difference being that it uses a corrected SG corresponding to the corrected Brix:
ap = 0.59Bcsgc (48)
where
Bc = corrected Brix = (Bi - 3)
sgc = SG corresponding to Bc
CEC Method
The official table for determining potential alcohol from Brix in the EU can be found in Table II (page 17) of The Official Journal of the European Communities (CEC, 1990). FermCalc interpolates values of potential alcohol from this table using the Brix value corresponding to the initial must SG.
Pambianchi Method
Daniel Pambianchi (2008) proposed the following formula to calculate a theoretical potential alcohol content:
ap = 0.55Bi - 0.63 (49)
Duncan & Acton Method
This method uses the Duncan & Acton SG drop equation assuming that the final SG (sgf) is 1.0:
ap = 1000(sgi - 1.0)/[7.75 - 3.75(sgi - 1.007)] (50)
Honneyman Method
William Honneyman (1966) developed the following equation for potential alcohol of musts prepared with cane sugar:
ap = 165.9sgi - 168.2 (51)
User Defined Method
In this method, the user supplies a factor f to be multiplied by the initial Brix of the must:
Back to top
ap = f·Bi (52)
OIML Calculator
Introduction
The OIML Calculator solves the general formula for calculating the densities of mixtures of ethanol and water found in International Recommendation 22: International Alcoholometric Tables by the International Organisation of Legal Metrology (OIML, 1973).
Input Field Definitions
Density – The density of the ethanol/water mixture. This field can be either an input field or an output field. If it is used as an input field, the alcohol content is calculated from the entered values of density and temperature. If alcohol content is entered, density is calculated. Range: 771.93 to 1000 kg/m3
Temperature – The temperature at which to calculate the density or alcohol content for the ethanol/water mixture. If the temperature is changed, either the density or alcohol content will be recalculated, depending on which one was entered last. Range: -20°C (-4°F) to 40°C (104°F)
Alcohol Content – The alcohol content of the ethanol/water mixture. This field can be either an input field or an output field. If it is used as an input field, the density is calculated from the entered values of alcohol content and temperature. If density is entered, alcohol content is calculated. Range: 0% to 100% vol/vol
Calculation Details
The OIML Calculator uses the general formula for calculating the densities of mixtures of ethanol and water found in International Recommendation 22: International Alcoholometric Tables by the International Organisation of Legal Metrology (OIML, 1973). The general formula has the form:
ρ = A1 + ΣiAipi-1 + ΣjBj(t-20)j + ΣmΣnCm,npn(t-20)m (53)
where
ρ = density of ethanol/water mixture, kg/m3
p = ethanol concentration by weight, fraction
t = temperature, ºC
A, B, C = coefficients
i = 2 to 12
j = 1 to 6
m = 1 to 5
n = 1 to 11
Given an alcohol content and a temperature, equation (53) can be solved directly for the density of the mixture. Given a density and a temperature, FermCalc calculates the associated alcohol content using an iterative technique.
Back to topDry Matter (Solids Content) Calculator
Introduction
The Dry Matter Calculator determines the solids content of a wine from its alcohol content and specific gravity. This is useful for winemakers who determine the alcohol content of their wines by other means, such as distillation or ebulliometry, and would like to calculate solids content. Two methods are used to determine the solids content:
The calculator will adjust the hydrometer reading for temperature if the measurement temperature and the hydrometer calibration temperature are provided. If the entered SG value is already corrected for temperature, or if temperature corrections are not desired, simply enter 20°C (68°F) in both of the temperature fields and no corrections will be made. Details of the hydrometer temperature correction calculations can be found here.
This calculation is only valid for SG or density measurements obtained with a hydrometer or some other device for directly measuring SG or density. It does not work with refractometer readings because of the obscuration effect of alcohol on refractive index.
Input Field Definitions
Alcohol Content – The alcohol content of the wine. Range: 0% to 100% vol/vol
Hydrometer SG Reading – The hydrometer SG reading for the wine sample. Range: 0.77193 to 1.55454
SG Reading Temperature – The temperature of the wine sample at the time of the SG reading. Range: 0°C (32°F) to 40°C (104°F)
Calibration Temperature – The hydrometer calibration temperature of the hydrometer used to take SG reading. Range: 0°C (32°F) to 40°C (104°F)
Output Field Definitions
Corrected SG (20°C/20°C) – The temperature-corrected SG value.
True Brix (Solids Content) – The total solids content of the wine calculated from alcohol content and the corrected SG value.
Calculation Details
OIV Method
The OIV method is described in the Compendium of International Methods of Analysis by the The International Organisation of Vine and Wine (OIV, 2009). This method assumes that solids (dry matter) cause the same increase in specific gravity in pure water as they do in wine. The specific gravity of the alcohol-free wine is calculated as follows:
sgr = sgv - sga + 1.0 (54)
where
sgr = SG of the alcohol-free wine
sgv = SG of the wine
sga = SG of a water-alcohol mixture with the same alcohol content as the wine
The value of sga in equation (54) is calculated from the alcohol content using the OIML formula. The solids content (true brix) is then calculated from the resulting value of sgr using the Brix conversion equation.
This method has a temperature basis of 20°C (68°F).
Hackbarth Method
This method utilizes the Hackbarth model described above, which allows calculation of SG given the alcohol and solids contents. In this case, we must solve the equations iteratively for solids content given the SG and alcohol content.
This method has a temperature basis of 20°C (68°F).
Back to top
© 2007-2021 Steve Gross
Last updated
11 September 2021.